The Fas antigen (Fas) belongs to the tumor necrosis factor (TNF)/nerve progress factor receptor family, and it mediates apoptosis. Using a soluble type of mouse Fas, ready by fusion with human immunoglobulin Fc, Fas ligand was detected on the cell floor of a cytotoxic T cell hybridoma, PC60-d10S. A cell inhabitants that extremely expresses Fas ligand was sorted utilizing a fluorescence-activated cell sorter, and its cDNA was remoted from the sorted cells by expression cloning.
The amino acid sequence indicated that Fas ligand is a sort II transmembrane protein that belongs to the TNF family. The recombinant Fas ligand expressed in COS cells induced apoptosis in Fas-expressing goal cells. Northern hybridization revealed that Fas ligand is expressed in activated splenocytes and thymocytes, in step with its involvement in T cell-mediated cytotoxicity and in a number of nonlymphoid tissues, resembling testis.
The normal semen evaluation is the first line and the hottest laboratory check in the analysis of male fertility. It evaluates sperm focus, motility, morphology and their vitality. However, it’s well-known that ordinary outcomes of semen evaluation can’t exclude males from the causes of {couples}’ infertility.
One of the most essential parameters of sperm in its fertilizing potential is “Sperm chromatin integrity” that has direct constructive correlation with Assisted Reproductive Techniques (ART) outcomes together with; fertilization charge, embryo high quality, being pregnant and profitable supply charge. It appears that sperm DNA chromatin integrity gives higher diagnostic and prognostic approaches than normal semen parameters. For these causes under-standing the sperm chromatin construction.
Fas ligand (FasL) is a member of the tumor necrosis factor family and induces apoptosis in Fas (CD95)-bearing goal cells. In this examine, we generated a number of mAbs that react with mouse FasL (mFasL) and characterised their useful properties. One of these mAbs, Ok10, particularly reacted with mFasL derived from C57BL/6 (B6) mice, however not that from BALB/c mice as estimated by floor staining and blocking of cytotoxic actions of mFasL transfectants, suggesting a polymorphism of mFasL. Sequence evaluation of mFasL cDNA from a number of strains revealed that BALB/c and DBA/2 mice have three nucleotide variations from the identified B6 and C3H sequences.
This report investigates the response of CD8(+) T cells to antigens introduced by B cells. When C57BL/6 mice had been injected with syngeneic B cells coated with the Kb-restricted ovalbumin (OVA) determinant OVA257-264, OVA-specific cytotoxic T lymphocyte (CTL) tolerance was noticed. To examine the mechanism of tolerance induction, in vitro-activated CD8(+) T cells from the Kb-restricted, OVA-specific T cell receptor transgenic line OT-I (OT-I cells) had been cultured for 15 h with antigen-bearing B cells, and their survival was decided. Antigen recognition led to the killing of the B cells and, surprisingly, to the dying of a massive proportion of the OT-I CTLs.
The chimeric receptors had been ready by exchanging the cytoplasmic area between leukemia inhibitory factor (LIF) receptor alpha subunit (gp190) and the different subunit-gp130 (190/130,130/190) and individually transduced into leukemia line HL-60 (to have the wild sort subunit).
Advantages of utilizing this mammalian expression system
The function is to research which subunit for activating MAPK p42/44 in leukemia cell whereas the cytoplasmic area homodimerization (190cyt-190cyt, 130cyt-130cyt) was induced by LIF. The outcomes confirmed that MAPK p42/44 expression degree after LIF stimulation 5 h was decrease in the transformants with pED 130/190 (190cyt- 190cyt) (p < 0.01) and greater in the transformants with pED 190/130 (130cyt- 130cyt) (p < 0.05) than these in the father or mother cells. Meanwhile, MAPK p42/44 phosphorylation (Thr202/Tyr204) The distal cytoplasmic motifs of leukemia inhibitory factor receptor α-chain (LIFRα-CT3) can independently induce intracellular myeloid differentiation in acute myeloid leukemia (AML) cells by gene transfection; nonetheless, there are important limitations in the potential medical use of these motifs as a consequence of liposome-derived genetic modifications.
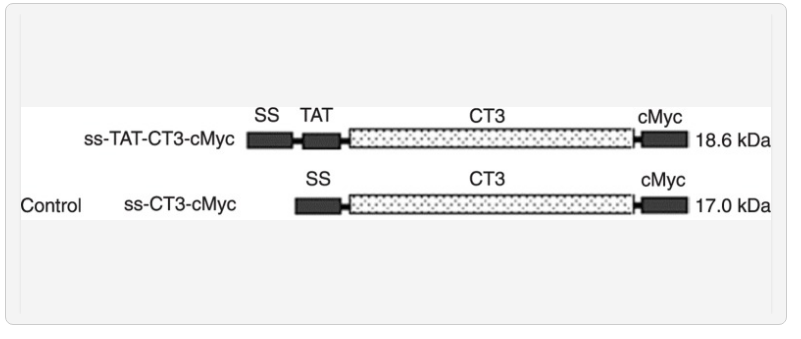
To produce a doubtlessly therapeutic LIFRα-CT3 with cell-permeable exercise, we constructed a eukaryotic expression pcDNA3.0-TAT-CT3-cMyc plasmid with a sign peptide (ss) inserted into the N-terminal that codes for an ss-TAT-CT3-cMyc fusion protein. The secure transfection of Chinese hamster ovary (CHO) cells through this vector and subsequent choice by Geneticin resulted in cell traces that specific and secrete TAT-CT3-cMyc.
The spent medium of pcDNA3.0-TAT-CT3-cMyc-transfected CHO cells might be purified utilizing a cMyc-epitope-tag agarose affinity chromatography column and might be detected through SDS-PAGE, with antibodies towards cMyc-tag. The direct administration of TAT-CT3-cMyc to HL-60 cell tradition media precipitated the enrichment of CT3-cMyc in the cytoplasm and nucleus inside 30 min and led to a important discount of viable cells (P < 0.05) eight h after publicity.
The benefits of utilizing this mammalian expression system embrace the ease of producing TAT fusion proteins which are adequately transcripted and the potential for a sustained manufacturing of such proteins in vitro for future AML remedy.
The distal cytoplasmic motifs of leukemia inhibitory factor receptor α-chain (LIFRα-CT3) can independently induce intracellular myeloid differentiation in acute myeloid leukemia (AML) cells by gene transfection; nonetheless, there are important limitations in the potential medical use of these motifs as a consequence of liposome-derived genetic modifications.
To produce a doubtlessly therapeutic LIFRα-CT3 with cell-permeable exercise, we constructed a eukaryotic expression pcDNA3.0-TAT-CT3-cMyc plasmid with a sign peptide (ss) inserted into the N-terminal that codes for an ss-TAT-CT3-cMyc fusion protein.
The secure transfection of Chinese hamster ovary (CHO) cells through this vector and subsequent choice by Geneticin resulted in cell traces that specific and secrete TAT-CT3-cMyc. The spent medium of pcDNA3.0-TAT-CT3-cMyc-transfected CHO cells might be purified utilizing a cMyc-epitope-tag agarose affinity chromatography column and might be detected through SDS-PAGE, with antibodies towards cMyc-tag.
The direct administration of TAT-CT3-cMyc to HL-60 cell tradition media precipitated the enrichment of CT3-cMyc in the cytoplasm and nucleus inside 30 min and led to a important discount of viable cells (P < 0.05) eight h after publicity.
The benefits of utilizing this mammalian expression system embrace the ease of producing TAT fusion proteins which are adequately transcripted and the potential for a sustained manufacturing of such proteins in vitro for future AML remedy.