Fas antigen is a cell-surface protein that mediates apoptosis. It is expressed in assorted tissues along with the thymus and has structural homology with lots of cell-surface receptors, along with tumour necrosis problem receptor and nerve growth problem receptor. Mice carrying the lymphoproliferation (lpr) mutation have defects in the Fas antigen gene.
The lpr mice develop lymphadenopathy and endure from a systemic lupus erythematosus-like autoimmune sickness, indicating an very important place for Fas antigen in the unfavourable alternative of autoreactive T cells in the thymus.
Somatic hypermutation significantly modifies rearranged
Somatic hypermutation significantly modifies rearranged immunoglobulin (Ig) genes in germinal coronary heart (GC) B cells. However, the bcl-6 gene may even buy somatic mutations in the course of the GC response, indicating that certain non-Ig genes could also be centered by the somatic hypermutation gear.
The CD95 gene, implicated in unfavourable alternative of B lymphocytes in GCs, is especially expressed by GC B cells and was simply currently acknowledged as a tumor suppressor gene being ceaselessly mutated in (publish) GC B cell lymphomas.
In this look at, the 5′ space (5’R) and/or the ultimate exon coding for the dying space (DD) of the CD95 gene have been investigated in naive, GC, and memory B cells from seven healthful donors. About 15% of GC and memory, nevertheless not naive, B cells carried mutations contained in the 5’R (mutation frequency 2.5 x 10(-4) per basepair).
Mutations contained in the DD have been very unusual nevertheless might probably be successfully chosen by inducing CD95-mediated apoptosis: in 22 apoptosis-resistant cells, 12 DD mutations have been found.
These outcomes level out that human B cells should buy somatic mutations of the CD95 gene in the course of the GC response, which most likely confers apoptosis resistance and can counteract unfavourable alternative by manner of the CD95 pathway.
Expression of the adenovirus E1A oncogene induces apoptosis which impedes every the transformation of main rodent cells and productive adenovirus an an infection of human cells. Coexpression of E1A with the E1B 19,000-molecular-weight protein (19Okay protein) or the Bcl-2 protein, every of which have antiapoptotic train, is necessary for atmosphere pleasant transformation.
Induction of apoptosis by E1A in rodent cells is mediated by the p53 tumor suppressor gene, and every the E1B 19Okay protein and the Bcl-2 protein can overcome this p53-dependent apoptosis.
The helpful similarity between Bcl-2 and the E1B 19Okay protein steered that they might act by comparable mechanisms and that Bcl-2 would possibly complement the requirement for E1B 19Okay expression all through productive an an infection. Infection of human HeLa cells with E1B 19Okay loss-of-function mutant adenovirus produces apoptosis characterised by enhanced cytopathic outcomes (cyt phenotype) and degradation of host cell chromosomal DNA and viral DNA (deg phenotype).
Failure to inhibit apoptosis outcomes in premature host cell dying, which impairs virus yield. HeLa cells categorical terribly low ranges of p53 as a consequence of expression of human papillomavirus E6 protein.
Levels of p53 have been significantly elevated by E1A expression all through adenovirus an an infection. Therefore, E1A would possibly induce apoptosis by overriding the E6-induced degradation of p53 and promoting p53 accumulation.
Stable Bcl-2 overexpression in HeLa cells contaminated with the E1B 19Okay- mutant adenovirus blocked the induction of the cyt and deg phenotypes. Expression of Bcl-2 in HeLa cells moreover conferred resistance to apoptosis mediated by tumor necrosis problem alpha and Fas antigen, which can be a longtime function of the E1B 19Okay protein.
Expression of the adenovirus E1A oncogene induces
A comparability of the amino acid sequences of Bcl-2 family members and that of the E1B 19Okay protein indicated that there was restricted amino acid sequence homology between the central conserved domains of E1B 19Okay and Bcl-2. This space of the E1B 19Okay protein is significant in transformation and regulation of apoptosis, as determined by mutational analysis.
The restricted sequence homology and helpful equivalency provided extra proof that the Bcl-2 and E1B 19Okay proteins would possibly possess related mechanisms of movement and that the E1B 19Okay protein would be the adenovirus equal of the cell Bcl-2 protein.
Huge apoptosis in T cells after repeated in vitro T cell receptor
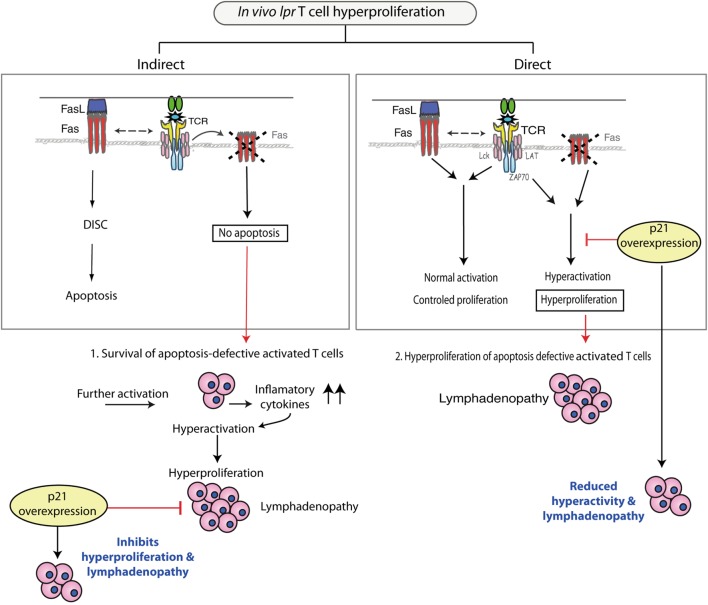
Fas induces large apoptosis in T cells after repeated in vitro T cell receptor (TCR) stimulation and is necessary for lymphocyte homeostasis in Fas-deficient (lpr) mice. Although the in vitro Fas apoptotic mechanism has been outlined, there is a large conceptual gap between this in vitro phenomenon and the pathway that outcomes in in vivo progress of lymphadenopathy and autoimmunity. A putting abnormality in lpr mice is the intense proliferation of CD4+ and CD8+ T cells, and further so of the double-negative TCR+CD4-CD8-B220+ T cells.
The basis of lpr T cell hyperproliferation stays elusive, as a result of it could actually’t be explained by Fas-deficient apoptosis. T cell-directed p21 overexpression reduces hyperactivation/hyperproliferation of all lpr T cell subtypes and lymphadenopathy in lpr mice. p21 controls progress of repeatedly stimulated T cells with out affecting apoptosis. These outcomes confirm a direct hyperlink